X-chromosome inactivation is a fascinating cellular process that plays a crucial role in managing genetic balance between genders, particularly in females who carry two copies of the X chromosome. This unique form of chromosomal silencing is vital for preventing the overexpression of genes linked to the X chromosome, thereby influencing various genetic disorders. Recent research led by Jeannie T. Lee at Harvard Medical School reveals how an RNA molecule known as Xist triggers this inactivation, presenting potential breakthroughs in the treatment of conditions like Fragile X Syndrome and Rett Syndrome. By unraveling the complexities of X-chromosome inactivation, scientists hope to pave the way for innovative therapeutic approaches that could reshape the landscape of genetic disorders treatment. As we delve deeper into understanding this mechanism, we also uncover new possibilities in chromosomal research breakthroughs that could benefit countless individuals affected by X-linked mutations.
The phenomenon of X-chromosome silencing, often referred to as the process of X-chromosome inactivation, entails a crucial biological adjustment that occurs in females possessing dual X chromosomes. This intricate mechanism ensures that gene dosage remains balanced, as only one X chromosome is active while the other remains dormant. Investigations into this form of gene regulation have unveiled significant insights into the role of Xist RNA, a key player in orchestrating the inactivation process. The implications of these studies stretch beyond academic curiosity, leading to promising avenues for the management of various genetic disorders, including Fragile X and Rett syndromes. As we explore alternative terminology and research in this domain, we continue to unlock the potential for groundbreaking therapies that address passenger mutations on the X chromosome.
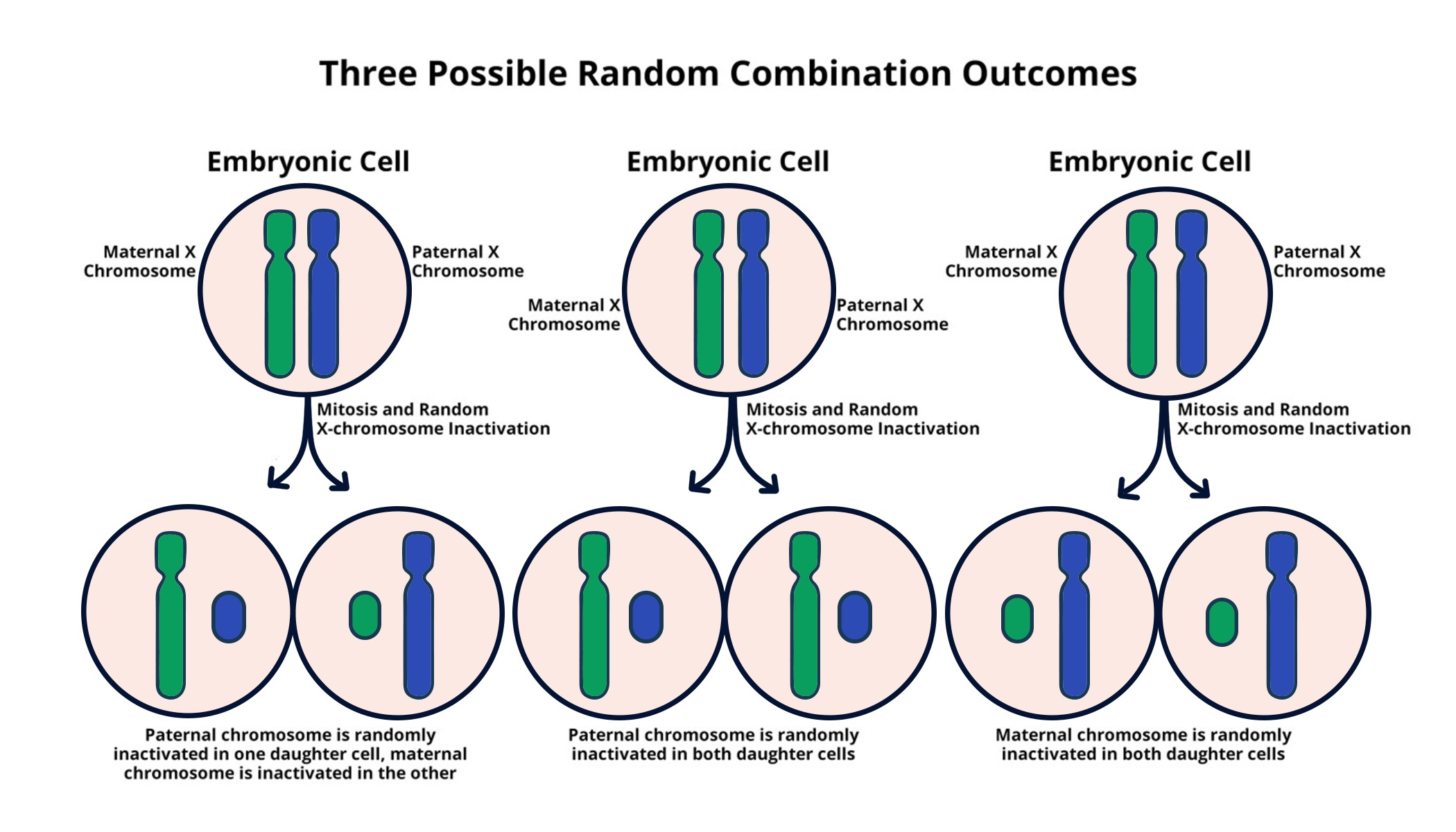
Understanding X-Chromosome Inactivation
X-chromosome inactivation is a biological mechanism crucial for balancing gene expression between males and females. In females, two X chromosomes are present, yet only one is actively used, while the other remains largely silenced. This process is vital to prevent the overexpression of X-linked genes, potentially leading to developmental issues. The intricacies of how X-inactivation occurs have fascinated researchers for decades, with significant contributions from pioneers like Jeannie T. Lee.
The mechanism of X-inactivation involves the Xist RNA molecule, a critical player that coats the X chromosome and alters the properties of the chromatin surrounding it. This transformation essentially turns the chromosome into an inactive state. As researchers delve deeper into this process, they uncover potential therapeutic avenues to address genetic disorders, including Fragile X Syndrome and Rett Syndrome, which disproportionately affect those with mutations on the X chromosome.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important in genetic disorders like Fragile X Syndrome?
X-chromosome inactivation is a biological process in female mammals where one of the two X chromosomes is inactivated to prevent overexpression of X-linked genes. This is crucial in genetic disorders such as Fragile X Syndrome, as mutations may exist on one X chromosome, and understanding this mechanism helps in developing potential treatments that could unsilence the healthy gene.
How does the Xist RNA molecule contribute to X-chromosome inactivation?
The Xist RNA molecule plays a pivotal role in X-chromosome inactivation by coating the inactive X chromosome and altering the surrounding chromatin’s material properties. This process effectively transforms the ‘Jell-O-like’ substance surrounding the chromosome, leading to its silencing, which is critical for maintaining gene balance in female cells.
What are the implications of X-chromosome inactivation research for treatments of Rett Syndrome?
Research on X-chromosome inactivation has significant implications for treating Rett Syndrome, as it reveals methods to potentially reactivate genes on the inactivated X chromosome. This could provide therapeutic avenues for individuals affected by this neurodevelopmental disorder, enabling access to the healthy version of mutated genes.
Can males benefit from treatments aimed at unsilencing X-linked genes despite not undergoing X-chromosome inactivation?
Yes, males can benefit from treatments that target unsilencing X-linked genes, as they can also have mutations affecting their single X chromosome. Understanding the processes behind X-chromosome inactivation provides insight into how similar mechanisms can address mutations in male carriers of genetic disorders like Fragile X Syndrome.
What recent breakthroughs in chromosomal research have been made regarding X-chromosome inactivation?
Recent breakthroughs in chromosomal research, particularly by Jeannie Lee’s lab, have uncovered how Xist RNA modifies the ‘Jell-O-like’ material surrounding the X chromosome, facilitating its inactivation. These findings not only advance our understanding of X-chromosome biology but also open new pathways for treating genetic disorders linked to mutations on the X chromosome.
| Key Points | Details |
|---|---|
| X-Chromosome Challenge | Females have two X chromosomes, while males have one, requiring inactivation of one copy in females. |
| Role of Xist | Xist is an RNA molecule that alters the properties of a gelatinous substance surrounding the X chromosome, facilitating inactivation. |
| Jell-O Analogy | The gelatinous material around chromosomes is compared to Jell-O, preventing chromosomes from tangling. |
| Therapeutic Potential | Research could lead to treatments for X-linked genetic disorders, such as Fragile X and Rett Syndromes. |
| Minimal Side Effects | Restoring function to mutated genes may not affect healthy genes, suggesting lower risk in therapies. |
| Long-term Research | It took decades of research to understand X-inactivation, with increasing realization of therapeutic possibilities. |
Summary
X-chromosome inactivation is a crucial cellular process that ensures dosage compensation between males and females. Recent breakthroughs by Jeannie T. Lee and her lab at Harvard Medical School have shed light on this complex mechanism, revealing the role of the RNA molecule Xist and a gelatinous substance that aids in silencing one of the X chromosomes in females. This understanding paves the way for potential therapies targeting genetic conditions linked to the X chromosome, such as Fragile X and Rett Syndromes. Furthermore, the ability to selectively reactivate mutated genes while preserving healthy ones offers promise for safe and effective treatments, marking a significant step forward in genetic research and therapeutic development.