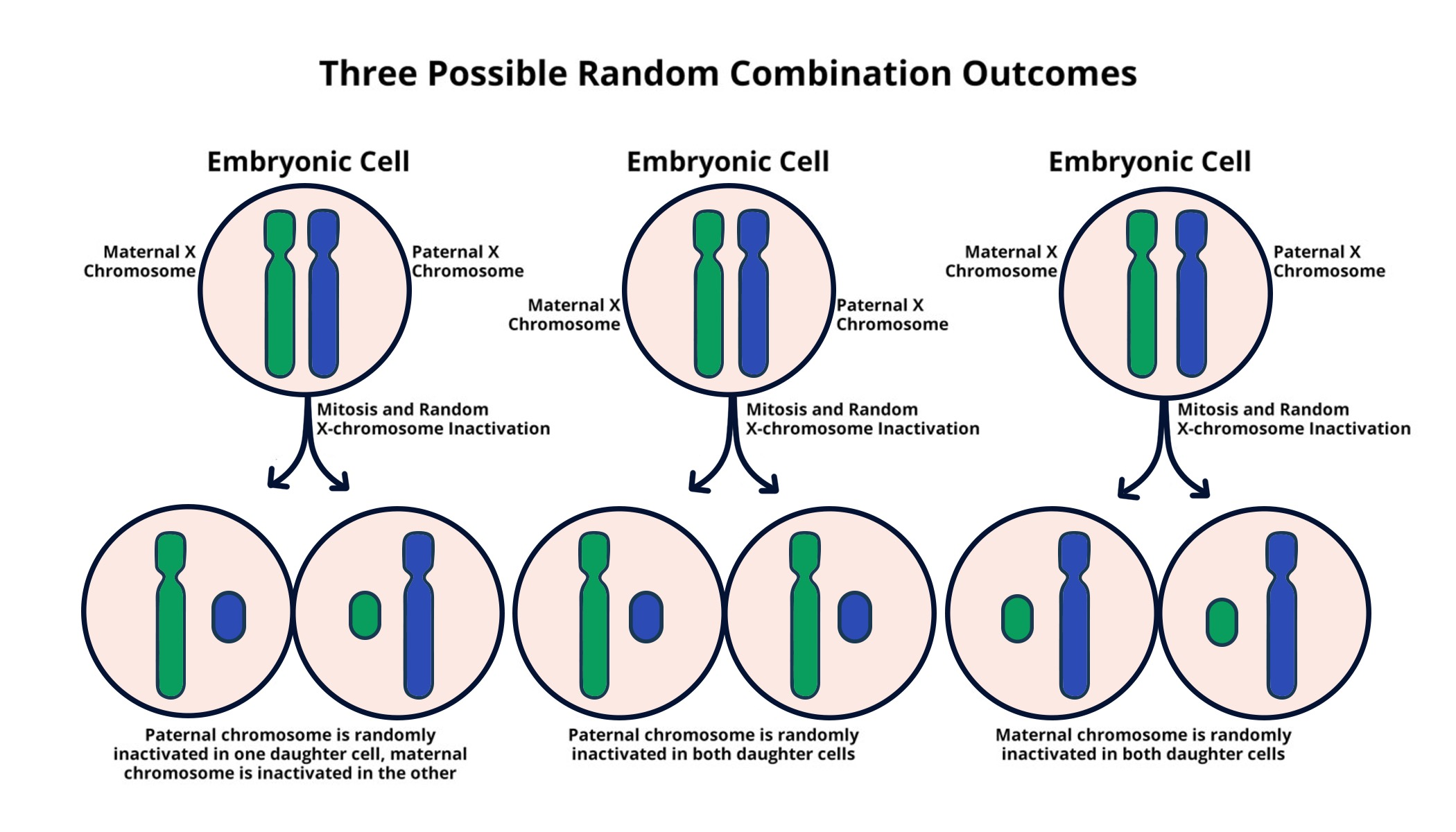
X chromosome inactivation is a fascinating and crucial biological process whereby one of a female’s two X chromosomes is rendered inactive to ensure that gene dosage remains balanced between the sexes. This intricate mechanism plays a significant role in the development of genetic diseases, including conditions such as Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. Understanding how this chromosomal silencing occurs could open up new avenues in gene therapy, potentially leading to groundbreaking treatments for these debilitating disorders. Recent research led by Jeannie T. Lee has shed light on the material properties of the cellular environment that facilitate this inactivation process, likening it to a gelatinous substance that separates chromosomes. With continued investigation into X chromosome inactivation, we inch closer to solving the genetic puzzles behind various chromosomal diseases and delivering effective therapeutic solutions.
The phenomenon of X chromosome inactivation, often referred to as XCI, plays a pivotal role in maintaining genetic equality between males and females. This process, essential for ensuring proper gene expression, particularly influences conditions caused by aberrations in X-linked genes, such as the debilitating Fragile X and neurodevelopmental Rett syndromes. By silencing one X chromosome in females, the cell achieves a balance that avoids gene overdose, a balance that becomes particularly critical in the context of gene therapy efforts aimed at rectifying mutations. Research into the mechanics of chromosomal silencing, akin to the fluid properties of Jell-O, reveals the complexities involved in gene regulation. Insights gained from this area could not only pave the way for innovative treatments but also enhance our knowledge of cellular biology and its implications for various genetic disorders.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process where one of the two X chromosomes in female mammals is silenced to prevent an overdose of X-linked gene products. This intricate mechanism not only helps maintain genetic balance but also plays a significant role in the functioning of genes across the genome. Research has shown that this inactivation is not just a simple shutdown; it involves complex interactions between the X chromosome and various molecular agents, including RNA molecules such as Xist, which play pivotal roles in the silencing process. By dissecting these interactions, scientists aim to better understand how genetic regulation can lead to the manifestation of certain X-linked genetic diseases such as Fragile X Syndrome and Rett Syndrome.
A better grasp of XCI may also open new avenues for effective gene therapy solutions. For instance, therapies that target the silencing mechanism of the X chromosome could potentially reactivate beneficial genes that have been historically ‘turned off’ due to mutations. These strategies could pave the way for restoring healthy gene function in conditions where one copy of the gene is mutated. Understanding the mechanics behind XCI not only sheds light on female-specific disorders but may also impact the treatment of males affected by X-linked diseases, highlighting the overarching significance of this genetic phenomenon.
The Role of Chromosomal Silencing in Genetic Therapy
Chromosomal silencing, particularly in the context of X chromosome inactivation, is of immense interest in the field of genetic therapy. The ability to silence or activate genes at will can lead to groundbreaking treatments for various genetic diseases, including those associated with the X chromosome. The discovery of how XCI manipulates the biophysical properties of nuclear material provides a framework for innovative therapeutic techniques. This understanding allows researchers to envision the possibility of developing treatments that could either disengage silenced genes or enhance gene expression where needed.
Gene therapy strategies that capitalize on the mechanics of chromosomal silencing could revolutionize how we approach genetic disorders. For instance, techniques aimed at reactivating the healthy form of genes locked away in inactivated alleles hold promise for clinical applications. As researchers like Jeannie T. Lee progress with their work, the prospect of employing chromosomal silencing mechanisms in clinical settings is not just a distant dream but a tangible goal that could immensely change the lives of individuals suffering from genetic disorders associated with mutations on the X chromosome.
Potential Therapies for Fragile X and Rett Syndromes
Fragile X Syndrome and Rett Syndrome are two prevalent conditions linked to mutations on the X chromosome, and recent advancements in research have sparked hope for new therapeutic interventions. Fragile X Syndrome, characterized by intellectual disabilities, is caused by a mutation in the FMR1 gene, while Rett Syndrome primarily affects girls and often leads to severe neurological impairments. The possibility of employing gene therapy to awaken the healthy allele trapped within an inactive X chromosome presents an opportunity to address these disorders at their genetic root. By understanding the mechanics of XCI, researchers can tailor treatment protocols that could reactivate or compensate for the dysfunctional forms of these genes.
Current studies being conducted by research teams are already showing promise in laboratory settings. Innovations in genetic manipulation that harness the potency of X chromosome inactivation are being tested to see how effectively they can restore gene function without causing adverse effects on other genes. As these research efforts advance toward clinical trials, the focus remains on ensuring safety and efficacy while targeting specific mutations—an approach that, if successful, could reshape the landscape of treatments for Fragile X and Rett syndromes.
Genome Editing: A New Frontier in Treating X-Linked Disorders
The rise of gene editing technologies such as CRISPR-Cas9 has ushered in a new era for genetic therapy, particularly for X-linked disorders. These precise editing techniques enable scientists to directly correct mutations that cause diseases like Fragile X Syndrome and Rett Syndrome. By leveraging the principles of X chromosome inactivation, researchers can not only target the faulty genes but also enhance understanding of how the X chromosome can be manipulated for therapeutic gain. Passage through the mechanisms of chromosomal silencing opens intriguing possibilities in genome editing—paving pathways to not only edit the genome effectively but also to potentially regenerate healthy gene function.
As gene editing matures, researchers are exploring how such interventions can work in tandem with X chromosome inactivation to achieve desired outcomes. The ability to modulate gene expression at multiple levels allows for tailored therapeutic approaches specific to each patient’s genetic makeup. This dual strategy may prove particularly beneficial in cases where simple correction of a mutation is insufficient for restoring full gene function due to the complexities of XCI. Ultimately, the convergence of genome editing and chromosomal silencing holds great potential for revolutionizing treatment paradigms for those living with X-linked genetic diseases.
Challenges in Reactivating Inactive X Chromosomes
Reactivating inactive X chromosomes presents a myriad of challenges, both scientifically and ethically. The mechanism of X-inactivation is complex, and while significant progress has been made in understanding how to manipulate this process, unforeseen complications arise, particularly in maintaining the delicate balance of gene expression. Initial successes in laboratory settings indicate that it’s possible to awaken silent genes, but the long-term effects of such reactivation remain largely unexplored. One critical consideration is ensuring that while we awaken certain genes, we do not inadvertently disrupt others that are crucial for normal cellular function.
Furthermore, the variability observed among patients adds another layer of complexity to the endeavor of developing therapies for genetic disorders linked to the X chromosome. Personal genetic backgrounds, co-existing conditions, and unique mutations complicate the landscape of gene reactivation. This unpredictability emphasizes the need for meticulous research and comprehensive clinical trials tailored to address the individual differences among patients, particularly those diagnosed with disorders like Fragile X Syndrome and Rett Syndrome. Overcoming these challenges is essential for transitioning from experimental approaches to reliable, widely available therapies.
The Future of X Chromosome Research
Research into X chromosome biology is poised for tremendous growth, driven by emerging technologies and growing understanding of genetic regulation. As more discoveries unfold about the intricacies of X chromosome inactivation, the potential for breakthrough therapies for a multitude of genetic disorders becomes clearer. The foundational work, spearheaded by dedicated researchers like Jeannie T. Lee, illustrates the vital connection between basic research and clinical application—a relationship that will define the future of genetic disease treatment.
The imminent integration of advanced genomic technologies, such as single-cell sequencing and high-resolution imaging, allows researchers to dissect the behavior of genes within the context of their chromosomal environment. These advancements enable a closer look at the mechanics involved in XCI and its implications for gene therapy. As the field moves forward, its ability to provide hope for individuals affected by X-linked disorders will continue to expand, inspiring the next generation of researchers to explore the unexplored territories of genetics.
Implications of Chromosomal Silencing for Male Patients
While chromosomal silencing is primarily associated with female biology due to the presence of two X chromosomes, understanding this process also carries implications for male patients affected by X-linked conditions. The mechanisms of gene silencing may help explain how mutations manifest in males, providing insights into potential treatment protocols that can address these challenges. Male patients with conditions like Fragile X Syndrome might benefit from therapies that harness similar principles of gene silencing and reactivation, despite having only one X chromosome. The nuances of XCI can inform approaches to gene therapy that target specific genetic mutations.
Moreover, researchers are investigating how silencing certain genes on the X chromosome affects males differently than females. Lessons learned from X chromosome research could lead to innovative treatment strategies that effectively target both male and female patients suffering from related disorders. As knowledge surrounding chromosomal silencing deepens, the potential to design multi-faceted therapies that meet the unique genetic needs of both sexes becomes increasingly feasible. Such advancements could signify a remarkable leap toward bridging the treatment gap for X-linked genetic disorders across populations.
The Role of RNA Molecules in Chromosomal Dynamics
RNA molecules play a critical role in the dynamic processes surrounding chromosomal behavior, particularly in X chromosome inactivation. The prominent RNA molecule, Xist, initiates the silencing process through its interaction with chromosomal material, effectively altering its local environment. This alteration is essential for the inactivation of one X chromosome in females, showcasing the fundamental role that RNA plays beyond its traditional function of coding for proteins. Understanding how RNA mediates interactions within chromosomal structures opens new avenues for targeted therapies aimed at disorders rooted in X-linked mutations.
Research continues to uncover various types of regulatory RNA that function alongside Xist, enhancing the complexity and effectiveness of chromosomal silencing. As scientists delve into the diverse roles of RNA in gene regulation, the potential for developing RNA-based therapies becomes apparent. Such therapeutic strategies would harness regulatory RNA’s natural mechanisms to combat genetic diseases like Fragile X Syndrome and Rett Syndrome, representing a transformative approach to treating disorders stemming from X chromosome mutations. The link between RNA dynamics and chromosomal behavior is one of the most exciting frontiers in genetic research.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases?
X chromosome inactivation is a biological process that occurs in females, where one of the two X chromosomes is randomly inactivated to ensure that gene dosage is balanced with males who have only one X chromosome. This process is crucial because improper X inactivation can lead to genetic diseases, such as Fragile X Syndrome and Rett Syndrome, caused by mutations on the X chromosome.
How does X chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is a genetic disorder linked to the X chromosome. In individuals with this syndrome, mutations affect the FMR1 gene. X chromosome inactivation can complicate the expression of the healthy version of this gene, as it may be silenced if it resides on the inactivated X chromosome. Unlocking this inactivated X chromosome holds promise for potential therapies.
What role does Xist RNA play in X chromosome inactivation?
Xist RNA is a key regulator of X chromosome inactivation. It is produced from the X chromosome and coats it, altering the biophysical properties of the surrounding chromosomal environment. This process is essential for the silencing of one of the X chromosomes in females, allowing for proper gene dosage and reducing the risk of genetic diseases.
Can X chromosome inactivation be targeted for gene therapy in Rett Syndrome?
Yes, targeting X chromosome inactivation shows potential for gene therapy in Rett Syndrome. Researchers, including those in Jeannie T. Lee’s lab, are developing methods to reactivate the healthy X chromosome to restore the function of genes that may be mutated, which could offer new treatment avenues for Rett Syndrome.
How does chromosomal silencing contribute to the treatment of genetic diseases like Fragile X Syndrome?
Chromosomal silencing through X chromosome inactivation can contribute to genetic disease treatments by allowing researchers to develop strategies to restore gene activity. By understanding how to un-silence the inactivated X chromosome, scientists aim to reactivate beneficial genes that are otherwise unavailable due to mutations, thus providing therapeutic options for conditions like Fragile X Syndrome.
What future advancements might arise from studying X chromosome inactivation?
Future advancements from studying X chromosome inactivation may include novel therapies for genetic diseases linked to the X chromosome, such as Fragile X and Rett Syndromes. Researchers like Jeannie T. Lee are exploring potential treatments that could trigger the reactivation of inactivated genes, paving the way for clinical trials and new avenues in gene therapy.
In what ways does X chromosome inactivation affect males with X-linked genetic disorders?
While males only have one X chromosome and do not undergo X chromosome inactivation, they still can be affected by mutations on that X chromosome. In cases like Fragile X Syndrome, even though males lack two X chromosomes, the silencing of certain genes can occur in their single X chromosome, impacting the manifestation of the disorder and indicating the need for therapeutic approaches that may focus on gene activation.
What challenges remain in the research of X chromosome inactivation and its connection to genetic disorders?
Despite advancements in understanding X chromosome inactivation, challenges remain, including comprehending how the reactivation of inactivated X chromosomes precisely restores gene function without affecting healthy genes. Ongoing research seeks to clarify these mechanisms to optimize potential therapies for genetic disorders linked to the X chromosome.
| Key Points |
|---|
| The X chromosome has a unique requirement for inactivation in females, as they have two copies while males have one. |
| Jeannie T. Lee’s lab has made significant contributions to understanding how X chromosome inactivation occurs, using a gelatinous substance that coats chromosomes. |
| The RNA molecule Xist plays a crucial role in modifying the properties of this gelatinous ‘Jell-O’, aiding in the segregation of the X chromosome. |
| Potential therapies being explored for genetic disorders linked to the X chromosome, specifically Fragile X Syndrome and Rett Syndrome. |
| The research may enable the activation of healthy genes from inactivated X chromosomes, offering a path to treat diseases without affecting other genes. |
Summary
X chromosome inactivation is a complex yet fascinating process essential for female cells, which possess two X chromosomes. As highlighted in the groundbreaking work of Jeannie T. Lee and her team, understanding this inactivation mechanism opens doors to innovative therapies for genetic disorders like Fragile X and Rett syndrome. Their research has uncovered the role of a gelatinous substance and the RNA molecule Xist in reorganizing chromosomal architecture, providing hope for improved treatment strategies. In conclusion, the study of X chromosome inactivation not only sheds light on fundamental biological questions but also paves the way for therapeutic advancements in genetic disease management.